What is Liquid Helium and why is it called a Superfluid?
“Liquid Helium”, at standard atmospheric pressures, refers to the physical state of helium at very low temperatures. When Helium is in this liquid state, we refer to it as “superfluid”. A superfluid is a state in which matter acts like a non-viscosity fluid. The substance behaves unlike any normal liquid,in that it passes over surfaces without any friction circulating within any container, subject only to its own inertia, through any and all obstacles or pores.
Liquid helium is known for its excellent thermal conductivity and its wide use in cryogenic applications (such as superconducting magnet cooling, scientific research and medicine). It is also used in industry for leak testing and in the manufacture of electronic and optical products
Abundance and Isotopes
There are two protons in the nucleus of each helium atom but there are isotopes of helium, as is the case for all elements. The identified helium isotopes contain between one and six neutrons, so they range in mass from 3 to 8. Although those with masses of 3 (helium-3 or 3 He) and 4 (helium-4 or 4He) are stabilized among these six isotopes, the others are all radioactive and deteriorate into other compounds very rapidly.
The helium onto earth is not a core element and is generated by nuclear fusion in the sun. The nuclei of isotope helium-4 are the nuclei of alpha particles expelled from the core of heavier radioactive compounds. Helium doesn’t collect in the environment in large amounts because the gravity of the Planet is not enough to stop it from flowing into space gradually. Helium-4is by far the more abundant of the two naturally occurring isotopes of helium: in atmospheric helium, helium-4 atoms are over 700,000:1, and in some minerals bearing helium, about 7,000,000:1.
Composition
It has an atomic number 2 chemical element. Its symbol is He and it has 4.0026 of standard atomic weight. It is in Group 18 of the Periodic Elements table, as it displays the properties of a noble gas with the total energy level. It also is a colourless, odourless, insipid, non-toxic gas, the less reactive element and doesn’t essentially form chemical compounds.
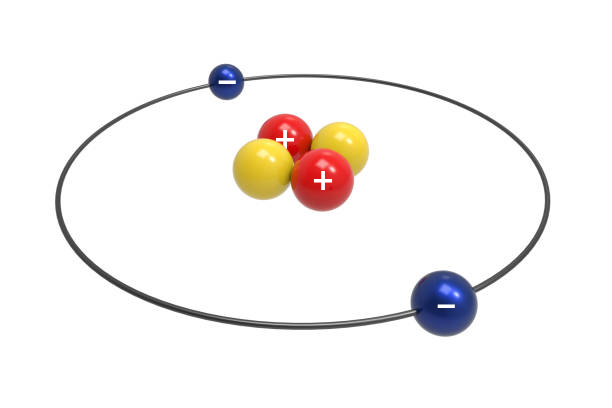
Distinction between the superfluid form of liquid helium 3 and helium 4
The superfluid form of liquid helium-4 happens at much higher temperatures than in helium-3. Every helium-4 molecule, due to its zero spin, is a boson-particle. Furthermore, helium 3 is a fermion particle that can only form bosons at temperatures far lower when combined, in a manner comparable to that in which electron pairing in superconductivity takes place.
Keep learning: The growing importance of the Liquified Natural Gas (LNG) industry
Principal characteristics of liquid helium
At absolute temperature, helium is so low that it takes on a liquid stater. The liquid boils at 4.2 K (−452°F or ‐269°C), a significantly lower temperature than any other substance, except hydrogen,, and below 2,172 K (−455.76°F) it has exceptional superfluidity characteristics, especially the possibility of flowing between small streams without any friction present.
Also, liquid helium is characterized by being inert and non-corrosive to most materials (so it can be used in differents applications). It has a low viscosity to flow easily. Finally, has good chemical stability and does not react easily with other chemical compounds.
Main advantages of using liquid helium
Liquid Helium has the greatest potential for ionization (24.587 eV). Helium is nearer to absolute zero than almost any other component so that the relatively low operating temperature and pressure of any refrigerant are achieved with liquid helium. Helium gas has a very high specific heat and thermal conductivity. At air pressure, Helium remains liquid to absolute zero and can only be solidified with 25 atm. Helium is exceptionally compressible through its solid form, allowing volume changes of more than 30%.
- Cryogenics (Current use of Liquid Helium): Cryogenics is the most common application of helium. The quantity of helium used by cryogenics in 1996 was approximately 620 million SCF. Magnet resonance imaging, semiconducting processes, as well as large and small-scale basic fieldwork requiring helium temperatures, are the primary cryogenic applications. However, the quantity used for each of the above purposes is not specifically provided.
- Magnetic Resonance Imaging (MRI): For magnetic resonance imaging (MRI) systems, Fluid Helium is mostly medically used. The superconductive magnets which are vital in so many additional appliances are supplied with liquid helium as a refrigerant. The affordability of helium and the stabilization of the market has lead to the fast development in public healthcare in the United States of MRI as a diagnostic tool. In the USA and around the world, there is a large and stable base of superconductive machines and this base could expand at 15% year-on-year.
- Aerostatics: Helium comes second after hydrogen on the periodic table (the second lightest component) and helium is below air density. Helium was therefore used during World War II as lift gas for balls, weather balloons, and aircraft. Helium would appear to be the preferred lifting gas, even though it isn’t witnessing the same boomas hydrogen since it is not combustible. The first application of helium since it was discovered on Earth was as a lifting gas. Hydrogen or a combination of hydrogen and nitrogen may be used to replace helium to produce lift.
- Semiconductor Processing: The main helium users of the semiconductor industry are silicon wafer vendors. The procedure of 12-in (300 mm) crystals is used to stabilize the hot balls of semiconductor material with a helium-cooled superconducting magnet. Plasma etching and vacuum pumping are other industrial processes that use helium. In each of the procedures recycling strategies to reduce helium consumption could be implemented. There is no knowledge of the total helium volume used by the semiconductor industry, however this key industry is clearly very valuable. The cost of helium may increase; however, semiconductor processing would be greatly impaired if helium disappears completely.
You may like: Uses and curiosities of liquid ethylene
Economic advantages liquid helium entails
At 200 atmospheres, helium in its gaseous state provides nearly five times the volume of the atmospheric pressure of its liquid state. The superconductive coils of resonances are used in laboratories. It has become a highly-valued product as a coolant in laboratories, chemistry generally requiring low temperatures. For the production of fiber optics, semiconductors and arc welding, helium is used for inert protective atmosphere. Helium can also be used for the detection of leakage in car air conditioning and can be used to inflate airbags after impact because it diffuses quickly.
But, as we said before, Cryogenics is the most common application of helium. At Cryospain we provide cryogenics engineering solutions that fit the specific demands of our clients: from turnkey projects to the supply of equipment, technical support, and maintenance. Would you like to know more about our services? Contact us.






 Kontaktieren Sie uns
Kontaktieren Sie uns